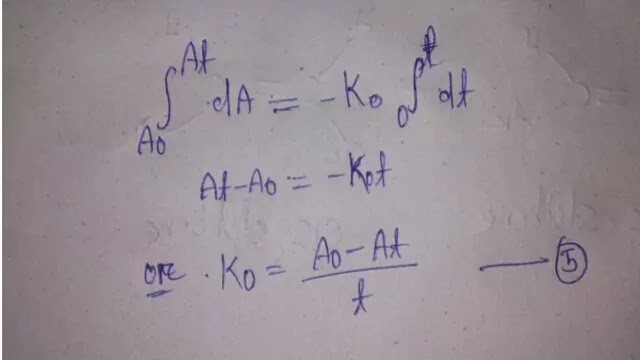Zero Order Reaction
Zero order reaction is defined as a reaction in which the rate does not depend on the concentration terms of the reactants.
This is mathematically expressed as:
-de =ko ---- (4)
dt
where k, is the specific rate constant for a zero order
Examples:
(1) Colour-loss of liquid multisulfonamide preparation. Colour-loss is proportional to decrease in the concentration.
(2) Oxidation of vitamin A in an oily solution.
(3) Photochemical degradation of chlorpromazine in aqueous solution.
Mechanism :
In zero order reaction, the rate must depend on some factor other than the concentration term. The rate limiting factors are solubility as in suspensions of absorption of light as iroto chemical reactions.
Derivation :
The rate equation for zero order can be written as:
-dA = ko ---- (4)
dt
where A is the absorbance (optical density) of the preparation.
In this reaction, the concentration is measured in terms of optical density. The negative sign indicates colour fading. If equation (4) is integrated, we get an equation that will permit the estimation of colour fading at any time, t.
Integrate equation (4) between initial absorbance, Ao at t = 0 time, and absorbance, At at t = t
Equation (5) is the integral equation for zero order reaction. In general, integral equation helps in estimating the reaction rate constant of any order. It also permits us to calculate the concentration of drug remain undecomposed after any time, t. Normally, the initial concentra tion is expressed as 'a' and the concentration at any time t, is 'e".
Then, equation (5) becomes
Ko = (a-c) -------(6)
t
Equation (6) may be written as
c = a - kol -------(7)
Equation (7) represents a linear expression, when c is plotted on y axis against on x axis. The line gives a negative slope and the magnitude is equal to ko A representative zero order plot is given in Figure 1-1a. The units for k, are conc/time. If the concentration is expressed as moles/liter, then k, will be moles/liter.sec. As a corollary, when the kinetic data is plotted in this fashion (Figure l-la), if a straight line is obtained, then, that reaction follows a zero order rate. The colour development during storage is also a sign of chemical reaction (instability). Still the order remains zero order. In this case, the slope is positive (Figure 1-1b).
In zero order, the absolute degradation remains same irrespective of the concentration. In other words, the higher the concentration, the more stable the solution will be. This is the advantage of the zero order. For example, the amount of vitamin A oxidised in a certain period of time is 300 units for a given dose of 3000 IU/ml. It means that the decrease in potency is 10 per cent. If the dose of vitamin is 3,00,000 IU/ml, then also the amount of vitamin oxidised will be 300 units only. Now the decrease in concentration is 0.1%. Therefore, by increasing the dose, the shelf life can be enhanced.
Figure 1-1a. A typical zero order plot for amount of substance unreacted (= absorbance) vs. time. Colour-fading indicating the decrease in absorbance (negative stope), drawn as per equation (7).
Figure 1-1b. A representative zero order plot of the amount of substance unreacted (= absorbance or optical density) vs. time. Colour-developement is indicated by increased absorbance (positive slope).
There are not many reactions that are truly zero order in the pharma- ceutical field. Most of those that appear to be zero order are, in fact, pseudo zero orders.
👉first Order Reaction, Order Of Reaction, Chemical Kinetics :- click here
👉Solid state decomposition, Order of Reaction, Chemical Kinetics :- click here
👉What is Half Life and Shelf Life, Order of Reaction, Chemical Kinetics :- click here
👉Apparent Zero Order Suspension, Chemical Kinetics, Physical Pharmaceutics :- click here
👉Zero Order Reaction, Chemical kinetics :- click here
👉ORDER OF REACTION, Chemical Kinetics Free PDF Note, Pharmacy Free PDF Book :- click here
👉ORDER OF REACTION, Chemical Kinetics Free PDF Note, Pharmacy Free PDF Book :- click here
👉MOLECULARITY OF REACTION :- click here
👉PRODRUG DESIGN :- click here
👉Application Of Prodrug :- click here
👉Classification Of Prodrug.:- click here
👉PRODRUGS, :- P.Valentina. Pharmacy PDF books for students free download :- click here
👉Chemical Kinetics, CVS Subrahmanyam full PDF file. bookhata free PDF books for students. :- click here
👉Metabolism And Extraction :- click here
👉What is Protein Binding :- click here
👉General Principle of Drug Action. Medicinal Chemistry Chapter-2 :- Click here
👉Semister–V, Medicinal Chemistry E-BOOK :- Click here
👉Pharmacognocy Semister-V, PDF Book :- Click here
👉Industrial Pharmacy-I PDF book, B-Pharm 5th Semister :- Click here
👉Interfacial Phenomena By CVS Subrahmanyam √ Physical Pharmaceutics √ 3rd Semister √ Pharmacy Free PDF Books Download :- Click here
👉Pharmaceutical Jurisprudence √ 5th Semister √ Pharmacy Free PDF Book Download :- click here
👉Pharmacology of drugs acting on cardio vascular system, 5th semister Pharmacology, Pharmacy Free PDF Book Download :- Click here
👉Autocoids and Related Drugs, Pharmacology, 5th Semister, Pharmacy Free PDF Book Download :- click here
👉Drug Stability, By CVS Subrahmanyam Physical Pharmaceutics, 4th Semister, Pharmacy Free PDF Book Download :- click here
👉Rheology, By CVS Subrahmanyam Physical Pharmaceutics, 4th Semister, Pharmacy Free PDF Book Download :- click here
👉Micromeritics, By CVS Subrahmanyam Physical Pharmaceutics, 4th Semister, Pharmacy Free PDF Books For Students Download :- click here
👉What is Absorption of Drug :- click here
👉What is Distribution Of Drug ? :- click here
👉Complexation and Protein Binding By CVS Subrahmanyam, 3rd Semister, Physical Pharmaceutics, Pharmacy Free PDF Book Download :- click here
👉Drug Metabolism, Med-Chem, 4th Semister, Bookhata Free PDF Books For Students download :- click here