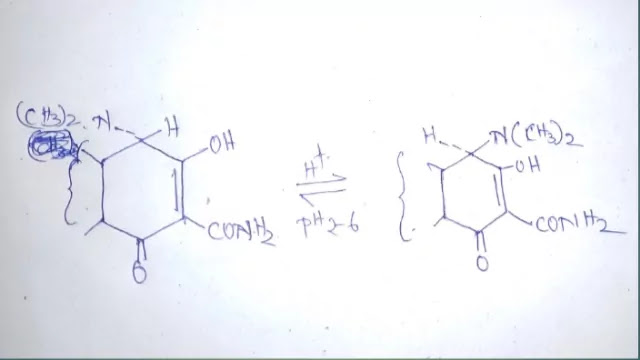
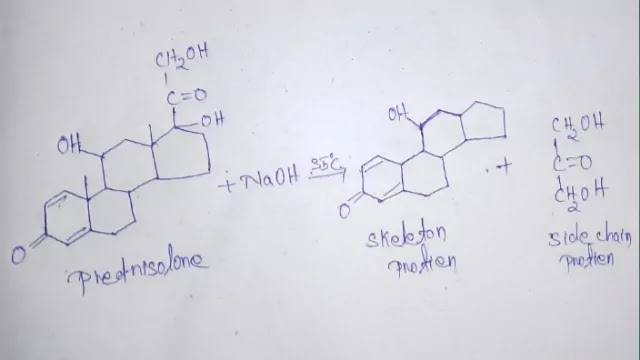
MOLECULARITY OF REACTION
Molecularity is defined in terms of a number which is equal to the number of molecules or atoms that must collide simultaneously to give the products.
The stoichiometric equation is essential to decide the molecularity of a reaction. Since molecularity is determined by postulating the mecha nism for a reaction, it must be a small number (integer), and cannot be a fraction or zero.
Unimolecular Reactions
In these reactions, molecularity is one, i.e., one type of molecules stoichiometrically participates in the reaction.
Example 1: Isomerisation of trans-stilbene to cis-stilbene.
Termolecular Reactions
Reactions of termolecular and other higher molecularity observed. This is because three or more molecules havi 6/33 kinetic energy must meet simultaneously in the same region u. -puj to yield a product. The occurrence of such an event is quite unlikely.
The rate equations for the above reactions can be written using the law of mass action. The concept of molecularity is of theoretical relevance, but in practice, order of a reaction is important, because it is obtained by experimentation.
👉first Order Reaction, Order Of Reaction, Chemical Kinetics :- click here
👉Solid state decomposition, Order of Reaction, Chemical Kinetics :- click here
👉What is Half Life and Shelf Life, Order of Reaction, Chemical Kinetics :- click here
👉Apparent Zero Order Suspension, Chemical Kinetics, Physical Pharmaceutics :- click here
👉Zero Order Reaction, Chemical kinetics :- click here
👉ORDER OF REACTION, Chemical Kinetics Free PDF Note, Pharmacy Free PDF Book :- click here
👉ORDER OF REACTION, Chemical Kinetics Free PDF Note, Pharmacy Free PDF Book :- click here
👉MOLECULARITY OF REACTION :- click here
👉PRODRUG DESIGN :- click here
👉Application Of Prodrug :- click here
👉Classification Of Prodrug.:- click here
👉PRODRUGS, :- P.Valentina. Pharmacy PDF books for students free download :- click here
👉Chemical Kinetics, CVS Subrahmanyam full PDF file. bookhata free PDF books for students. :- click here
👉Metabolism And Extraction :- click here
👉What is Protein Binding :- click here
👉General Principle of Drug Action. Medicinal Chemistry Chapter-2 :- Click here
👉Semister–V, Medicinal Chemistry E-BOOK :- Click here
👉Pharmacognocy Semister-V, PDF Book :- Click here
👉Industrial Pharmacy-I PDF book, B-Pharm 5th Semister :- Click here
👉Interfacial Phenomena By CVS Subrahmanyam √ Physical Pharmaceutics √ 3rd Semister √ Pharmacy Free PDF Books Download :- Click here
👉Pharmaceutical Jurisprudence √ 5th Semister √ Pharmacy Free PDF Book Download :- click here
👉Pharmacology of drugs acting on cardio vascular system, 5th semister Pharmacology, Pharmacy Free PDF Book Download :- Click here
👉Autocoids and Related Drugs, Pharmacology, 5th Semister, Pharmacy Free PDF Book Download :- click here
👉Drug Stability, By CVS Subrahmanyam Physical Pharmaceutics, 4th Semister, Pharmacy Free PDF Book Download :- click here
👉Rheology, By CVS Subrahmanyam Physical Pharmaceutics, 4th Semister, Pharmacy Free PDF Book Download :- click here
👉Micromeritics, By CVS Subrahmanyam Physical Pharmaceutics, 4th Semister, Pharmacy Free PDF Books For Students Download :- click here
👉What is Absorption of Drug :- click here
👉What is Distribution Of Drug ? :- click here
👉Complexation and Protein Binding By CVS Subrahmanyam, 3rd Semister, Physical Pharmaceutics, Pharmacy Free PDF Book Download :- click here
👉Drug Metabolism, Med-Chem, 4th Semister, Bookhata Free PDF Books For Students download :- click here