APPLICATION OF PRODRUG
There are three main reasons for drug action, namely the pharmaceutical, the pharmacokinetic and pharmacodynamic phases. Problems exist in all the three phases but prodrug formation seeks to the problem in pharmaceutical and pharmacokinetic phases
1. Pharmaceutical Application
a) Improvement of taste
b) Improvement of odor
c) Reduction of GI irritation
d) Change of physical form of drug
e) Reduction of pain on injection
f) Enhancement of drug solubility
g) To improve chemical stability
2. Pharmacokinetic Application
a) Prolonged duration of action
b) Site specific drug design
c) Reduction of toxicity and adverse effect
d) To improve membrane transport
1. PHARMACEUTICAL APPLICATIONS
(a) Improvement of Taste
One of the reasons for poor patient compliance particularly in case of children is the bitterness, acidity or basicity of the drug. Two approaches can be utilized to overcome the bad taste of drug. The first is reduction of drug solubility in saliva and the other is to lower the affinity of drug towards taste receptor.
e.g Chloramphenicol has a bitter taste, so it is not well accepted by children. The palmitate ester of it is less soluble in saliva, so it masks the bitter taste.
(b) Improvement of odor
The odor of a compound depends upon its vapor pressure, a liquid with high vapor pressure will have a strong odor. For example Ethyl mercaptan is a foul smelling liquid used in the treatment of leprosy. This is converted to phthalate ester, diethyl dithioisophthalate that has higher boiling point and is odorless.
(c) To reduce Gastrie irritation
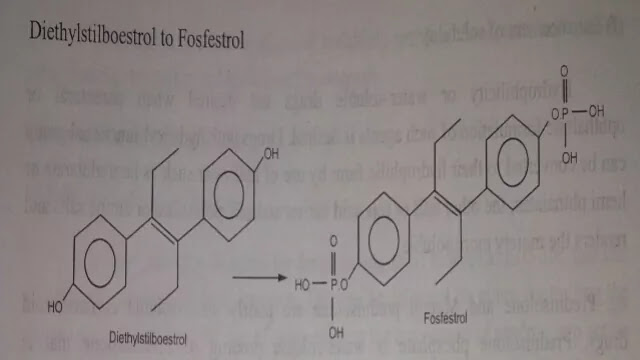
Several drugs (NSAIDS. Nicotinic acid, Kanamycin, Diethylstilboestrol) cause irritation and damage to gastric mucosa. Examples of prodrug designed to overcome such problems of gastric distress are given below.
Salicylic acid to Aspirin
Diethylstilbosterol to Fasofestrol
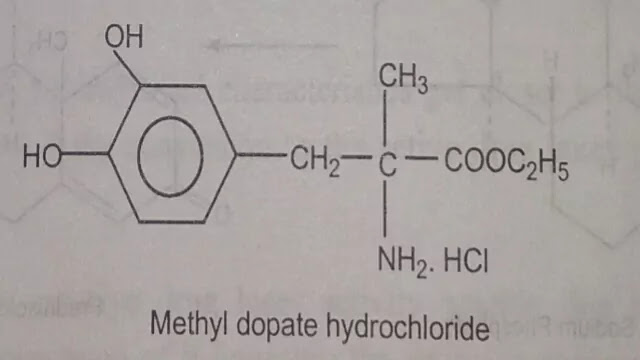
(d) Change of physical form of the drug
Some drug exists in a form, which is unsuitable for formulation. For example Methyldopa is a Zwitter ion and although charged, its crystal structure causes it to be only poorly water-soluble. In order to formulate this drug for injection which is converted into ethyl ester this may be formulated as the hydrochloride salt.
Methyl dopate hydrochloride
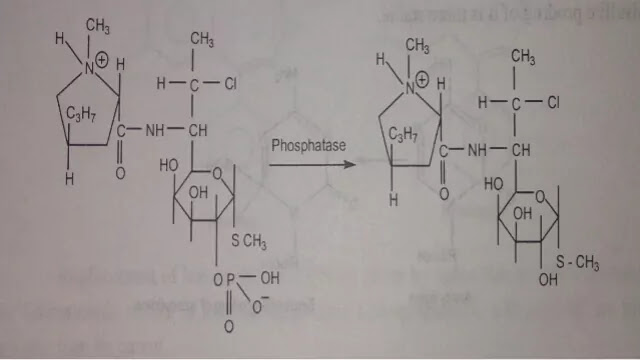
(e) Reduction of pain on injection
Intramuscular injection is particularly painful when drug precipitates or penetrates into the surrounding cell or when the solution is strongly acidic, alkaline or alcoholic. For example Clindamycin hydrochloride causes irritation when given by intramuscular route. This can be overcome by use of more water-soluble prodrug Clindamycin - 2' - phosphate.
(f) Enhancement of solubility
Hydrophilieity or water-soluble drugs are desired when parenteral or ophthalmic formulation of such agents is desired. Drugs with hydroxyl functional group can be converted to their hydrophilic form by use of half ester such as hemiglutarate or hemi phthalates; the other half of this acid carries sodium, potassium or amine salts and renders the moiety more soluble.
eg. Prednisolone and Methyl prednisolone are poorly water-soluble corticosteroid drugs. Prednisolone phosphate is water-soluble prodrug of Prednisolone that is activated invivo by phosphatase.
(g) To Improve Chemical Stability
Several drugs may decompose in their shelf life or in the GIT when used orally. To improve their stability prodrug approach is a good technique.
An antineoplatic drug Azacytidine hydrolyses readily in acidic pH but the bisulfite prodrug of it is more stable.
Erythromycin also has similar acid instability problem. Its stearate and estolate ester prodrugs are stable and hydrolyzed in stomach.
2. PHARMACOKINETIC APPLICATIONS
(i) Prolonged duration of action
Frequent dosing is required for drugs having short biological half-life; this can also be overcome by prodrug approach. This can be achieved by either controlling the release rate of drug from the site of application or the conversion of prodrug into active drug. For example, the diester of Pilocarpine has prolonged therapeutic effect than the Pilocarpine. The diester of the drug when applied as ophthalmic solution showed better intraocular penetration due to improved lipophilicity, and slow conversion of ester prodrug to active Pilocarpine prolong the activity,
a) Prolonged Activity
The prodrug by its improved characteristics get closer to the receptor site for longer period of time and the conversion to the active drug takes place at the site of action.
Nordazepam, a sedative drug loses activity quickly due to metabolism and excretion. A prodrug diazepam of it improves the retention characteristics, due to the presence of N-methyl group. Slow release of the Nordazepam in the liver by demethylation prolongs the activity.
b) Reduction of Toxicity and Adverse effect
An important objective of drug design is to develop drug with high activity and low toxicity, for example side effects such as gastric distress with NSAIDS have overcome by prodrug design have been discussed already.
Another example is epinephrine a drug used in the treatment of glaucoma. The drug has only limited local use because of poor intraocular penetration. But higher doses of it cause irritation of eyes and undesirable cardiovascular effects. The ester prodrug Dipivefrin increases lipophilicity, which has better intraocular penetration, and thus side effects are limited.
c) To Improve Membrane Transport
Interference with transport characteristic can serve many purposes. The introduction of a hydrophilic moiety can restrict a drug to the gastrointestinal tract and prevent its absorption. For eg.
Succinyl sulfathiazole.
On the other hand introduction of lipophilic group decreases the rate of hydrolysis, which liberates the active drug slowly, in depot preparation for a period of days or weeks. The antimalarial Cycloguanil pamoate can deliver the drug Cycloguanil for several months.
The membrane transport characteristic of the neurotransmitter dopamine used for the treatment of Parkinson's disease can be improved by administering its prodrug 3, 4 - Dihydroxy phenylalanine (Levodopa). This derivative has better blood-brain permeation characteristic since it uses amino acid channels for transportation. Once it enters the cell, decarboxylase enzyme removes the acid group to generate dopamine.

👉first Order Reaction, Order Of Reaction, Chemical Kinetics :- click here
👉Solid state decomposition, Order of Reaction, Chemical Kinetics :- click here
👉What is Half Life and Shelf Life, Order of Reaction, Chemical Kinetics :- click here
👉Apparent Zero Order Suspension, Chemical Kinetics, Physical Pharmaceutics :- click here
👉Zero Order Reaction, Chemical kinetics :- click here
👉ORDER OF REACTION, Chemical Kinetics Free PDF Note, Pharmacy Free PDF Book :- click here
👉ORDER OF REACTION, Chemical Kinetics Free PDF Note, Pharmacy Free PDF Book :- click here
👉MOLECULARITY OF REACTION :- click here
👉PRODRUG DESIGN :- click here
👉Application Of Prodrug :- click here
👉Classification Of Prodrug.:- click here
👉PRODRUGS, :- P.Valentina. Pharmacy PDF books for students free download :- click here
👉Chemical Kinetics, CVS Subrahmanyam full PDF file. bookhata free PDF books for students. :- click here
👉Metabolism And Extraction :- click here
👉What is Protein Binding :- click here
👉General Principle of Drug Action. Medicinal Chemistry Chapter-2 :- Click here
👉Semister–V, Medicinal Chemistry E-BOOK :- Click here
👉Pharmacognocy Semister-V, PDF Book :- Click here
👉Industrial Pharmacy-I PDF book, B-Pharm 5th Semister :- Click here
👉Interfacial Phenomena By CVS Subrahmanyam √ Physical Pharmaceutics √ 3rd Semister √ Pharmacy Free PDF Books Download :- Click here
👉Pharmaceutical Jurisprudence √ 5th Semister √ Pharmacy Free PDF Book Download :- click here
👉Pharmacology of drugs acting on cardio vascular system, 5th semister Pharmacology, Pharmacy Free PDF Book Download :- Click here
👉Autocoids and Related Drugs, Pharmacology, 5th Semister, Pharmacy Free PDF Book Download :- click here
👉Drug Stability, By CVS Subrahmanyam Physical Pharmaceutics, 4th Semister, Pharmacy Free PDF Book Download :- click here
👉Rheology, By CVS Subrahmanyam Physical Pharmaceutics, 4th Semister, Pharmacy Free PDF Book Download :- click here
👉Micromeritics, By CVS Subrahmanyam Physical Pharmaceutics, 4th Semister, Pharmacy Free PDF Books For Students Download :- click here
👉What is Absorption of Drug :- click here
👉What is Distribution Of Drug ? :- click here
👉Complexation and Protein Binding By CVS Subrahmanyam, 3rd Semister, Physical Pharmaceutics, Pharmacy Free PDF Book Download :- click here
👉Drug Metabolism, Med-Chem, 4th Semister, Bookhata Free PDF Books For Students download :- click here